Learning Outcomes:
i. Understand the unique stability of benzene due to its aromatic character.
ii. Compare the reactivity of benzene with alkanes and alkenes towards electrophilic addition reactions.
iii. Analyze the factors influencing the regioselectivity of electrophilic substitution reactions in benzene.
iv. Explain the mechanism of electrophilic aromatic nitration and bromination of benzene.
v. Appreciate the importance of understanding the reactivity of benzene in organic synthesis and industrial processes.
Introduction:
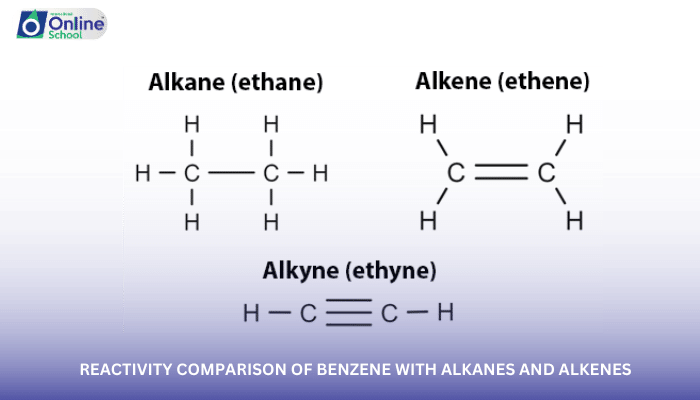
In the realm of organic chemistry, benzene stands as a cornerstone, captivating scientists with its unique stability and fascinating reactivity patterns. Unlike alkanes and alkenes, which readily undergo electrophilic addition reactions, benzene exhibits remarkable resistance to these reactions. This lesson delves into the comparative reactivity of benzene with alkanes and alkenes, exploring the factors that govern its unique behavior.
i. Benzene's Aromatic Stability: A Shield Against Addition
The exceptional stability of benzene arises from its aromatic character, a consequence of its continuous ring of overlapping pi orbitals. This delocalization of pi electrons stabilizes the molecule and makes it less susceptible to electrophilic addition reactions, which typically involve the attack of electrophilic species (electron-deficient particles) on the pi bonds of alkenes.
ii. Reactivity Comparison: Benzene Versus Alkanes and Alkenes
Alkanes, characterized by their saturated carbon-carbon single bonds, exhibit minimal reactivity due to the strong sigma (σ) bonds between carbon atoms. Alkenes, on the other hand, readily undergo electrophilic addition reactions due to the presence of the pi bond, which is more susceptible to attack by electrophilic species.
Benzene, with its aromatic stabilization, stands distinct from both alkanes and alkenes. While alkanes and alkenes undergo electrophilic addition reactions, benzene generally resists these reactions. This difference in reactivity highlights the unique aromatic nature of benzene.
iii. Electrophilic Substitution Reactions: Adding to Benzene with a Twist
While benzene is resistant to electrophilic addition, it does undergo electrophilic substitution reactions, where an electrophilic species replaces a hydrogen atom on the benzene ring. The regioselectivity, or the preferred position of substitution, is influenced by the electron distribution within the benzene ring.
iv. Factors Influencing Regioselectivity in Electrophilic Substitution
Directing Effect of Substituents: Electron-donating substituents (e.g., alkyl groups) direct electrophilic attack to the ortho and para positions, while electron-withdrawing substituents (e.g., halogens) direct attack to the meta position.
Inherent Reactivity of Substituents: Substituents with higher inherent reactivity, such as halogens, undergo electrophilic substitution more readily than those with lower reactivity, such as alkyl groups.
v. Mechanisms of Electrophilic Aromatic Nitration and Bromination
Electrophilic Aromatic Nitration: Nitric acid (HNO3) is protonated by sulfuric acid (H2SO4) to form the nitronium ion (NO2+), the electrophilic species. The nitronium ion attacks the benzene ring, forming a benzenonium ion intermediate, which upon deprotonation yields nitrobenzene.
Electrophilic Aromatic Bromination: Bromine (Br2) is activated by the presence of a Lewis acid catalyst, such as aluminum bromide (AlBr3), to form the bromine cation (Br+), the electrophilic species. The bromine cation attacks the benzene ring, forming a benzenonium ion intermediate, which upon deprotonation yields bromobenzene.
The unique reactivity of benzene, characterized by its resistance to electrophilic addition and its susceptibility to electrophilic substitution, highlights the profound influence of aromatic character on the behavior of molecules. Understanding these reactivity patterns is essential for organic synthesis, industrial processes, and comprehending the diverse chemistry of aromatic compounds.